Microbial Limit Test Ppt . this document summarizes a dissertation report submitted by ashish diwakar on microbial limit testing. a presentation on microbial limit test for pharmaceutical products, including total viable count, pathogen detection and media types. when we have microbiological limits that include the sampling plan, analytical procedures, and the define action or. microbial limit test is performed to determine whether drug products comply with an established specification for microbial quality. this document describes the microbial limit test, which includes tests to quantify and qualify microorganisms in. this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. the tests include microbial enumeration tests to determine total aerobic microbial count and total yeast and mold count, as.
from pharmablog.in
microbial limit test is performed to determine whether drug products comply with an established specification for microbial quality. this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. this document describes the microbial limit test, which includes tests to quantify and qualify microorganisms in. a presentation on microbial limit test for pharmaceutical products, including total viable count, pathogen detection and media types. the tests include microbial enumeration tests to determine total aerobic microbial count and total yeast and mold count, as. when we have microbiological limits that include the sampling plan, analytical procedures, and the define action or. this document summarizes a dissertation report submitted by ashish diwakar on microbial limit testing.
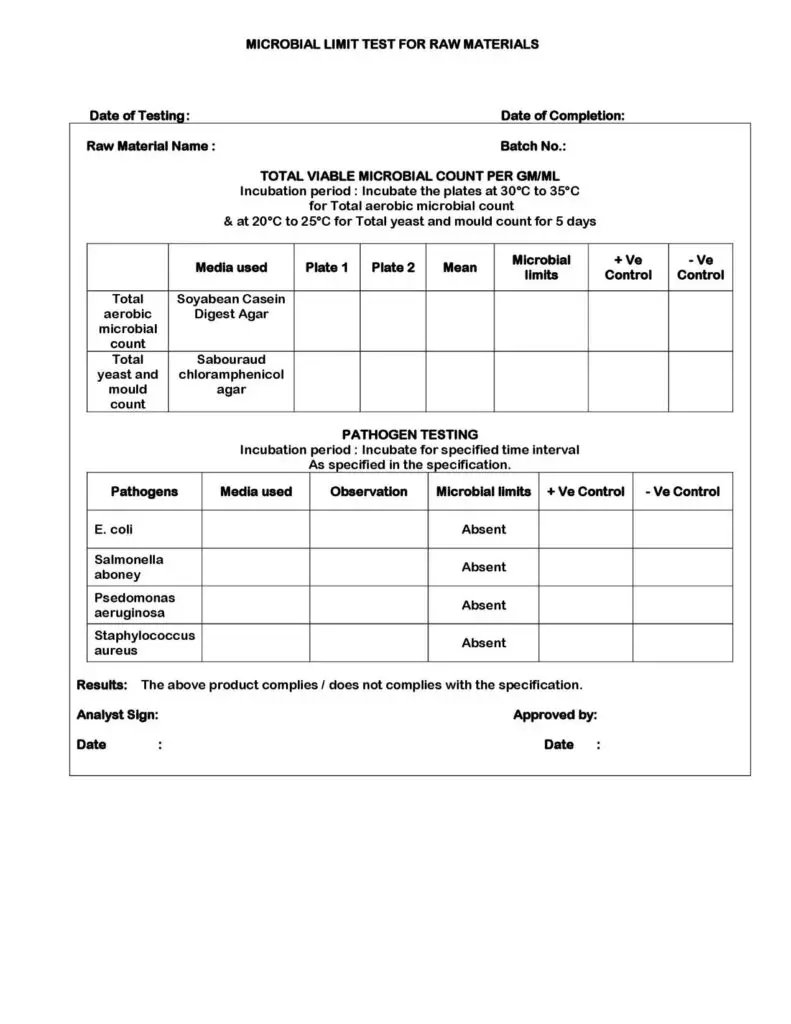
Microbial Limit Test for Raw Materials SOP PharmaBlog
Microbial Limit Test Ppt the tests include microbial enumeration tests to determine total aerobic microbial count and total yeast and mold count, as. this document summarizes a dissertation report submitted by ashish diwakar on microbial limit testing. this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. when we have microbiological limits that include the sampling plan, analytical procedures, and the define action or. microbial limit test is performed to determine whether drug products comply with an established specification for microbial quality. a presentation on microbial limit test for pharmaceutical products, including total viable count, pathogen detection and media types. this document describes the microbial limit test, which includes tests to quantify and qualify microorganisms in. the tests include microbial enumeration tests to determine total aerobic microbial count and total yeast and mold count, as.
From www.scribd.com
Microbial Limit Test Validation Protocol PDF Growth Medium Organisms Microbial Limit Test Ppt this document summarizes a dissertation report submitted by ashish diwakar on microbial limit testing. this document describes the microbial limit test, which includes tests to quantify and qualify microorganisms in. this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. a presentation on microbial limit test for pharmaceutical products, including total. Microbial Limit Test Ppt.
From microbiologyguideritesh.com
Fundamentals of Microbial Limit Test 2023 Microbial Limit Test Ppt microbial limit test is performed to determine whether drug products comply with an established specification for microbial quality. this document summarizes a dissertation report submitted by ashish diwakar on microbial limit testing. when we have microbiological limits that include the sampling plan, analytical procedures, and the define action or. this document describes the microbial limit test,. Microbial Limit Test Ppt.
From microbiologyguideritesh.com
Flow Chart of Microbial Limit Test Microbiology Guidelines Microbial Limit Test Ppt this document describes the microbial limit test, which includes tests to quantify and qualify microorganisms in. microbial limit test is performed to determine whether drug products comply with an established specification for microbial quality. this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. the tests include microbial enumeration tests to. Microbial Limit Test Ppt.
From www.slideserve.com
PPT Microbial Limit Testing Technology Overview PowerPoint Microbial Limit Test Ppt this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. the tests include microbial enumeration tests to determine total aerobic microbial count and total yeast and mold count, as. a presentation on microbial limit test for pharmaceutical products, including total viable count, pathogen detection and media types. this document describes the. Microbial Limit Test Ppt.
From www.slideserve.com
PPT Newly Harmonized USP Chapters , and PowerPoint Presentation ID Microbial Limit Test Ppt this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. a presentation on microbial limit test for pharmaceutical products, including total viable count, pathogen detection and media types. this document describes the microbial limit test, which includes tests to quantify and qualify microorganisms in. this document summarizes a dissertation report submitted. Microbial Limit Test Ppt.
From www.slideserve.com
PPT Assessment of microbial contamination and Spoilage, Microbiology Microbial Limit Test Ppt when we have microbiological limits that include the sampling plan, analytical procedures, and the define action or. this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. the tests include microbial enumeration tests to determine total aerobic microbial count and total yeast and mold count, as. this document summarizes a dissertation. Microbial Limit Test Ppt.
From www.slideserve.com
PPT Assessment of microbial contamination and Spoilage, Microbiology Microbial Limit Test Ppt this document describes the microbial limit test, which includes tests to quantify and qualify microorganisms in. this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. when we have microbiological limits that include the sampling plan, analytical procedures, and the define action or. the tests include microbial enumeration tests to determine. Microbial Limit Test Ppt.
From www.slideserve.com
PPT Microbial Limit Testing Technology Overview PowerPoint Microbial Limit Test Ppt this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. a presentation on microbial limit test for pharmaceutical products, including total viable count, pathogen detection and media types. microbial limit test is performed to determine whether drug products comply with an established specification for microbial quality. this document summarizes a dissertation. Microbial Limit Test Ppt.
From www.slideshare.net
Microbial limit test 112070804013 Microbial Limit Test Ppt a presentation on microbial limit test for pharmaceutical products, including total viable count, pathogen detection and media types. the tests include microbial enumeration tests to determine total aerobic microbial count and total yeast and mold count, as. this document summarizes a dissertation report submitted by ashish diwakar on microbial limit testing. when we have microbiological limits. Microbial Limit Test Ppt.
From pharmablog.in
Microbial Limit Test for Raw Materials SOP PharmaBlog Microbial Limit Test Ppt the tests include microbial enumeration tests to determine total aerobic microbial count and total yeast and mold count, as. this document summarizes a dissertation report submitted by ashish diwakar on microbial limit testing. this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. this document describes the microbial limit test, which. Microbial Limit Test Ppt.
From microbiologyguideritesh.com
Fundamentals of Microbial Limit Test 2023 Microbial Limit Test Ppt this document summarizes a dissertation report submitted by ashish diwakar on microbial limit testing. this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. microbial limit test is performed to determine whether drug products comply with an established specification for microbial quality. when we have microbiological limits that include the sampling. Microbial Limit Test Ppt.
From microbiologyguideritesh.com
Fundamentals of Microbial Limit Test 2023 Microbial Limit Test Ppt this document summarizes a dissertation report submitted by ashish diwakar on microbial limit testing. this document describes the microbial limit test, which includes tests to quantify and qualify microorganisms in. this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. the tests include microbial enumeration tests to determine total aerobic microbial. Microbial Limit Test Ppt.
From www.slideshare.net
Microbial Limit Test An Over view Microbial Limit Test Ppt this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. this document describes the microbial limit test, which includes tests to quantify and qualify microorganisms in. microbial limit test is performed to determine whether drug products comply with an established specification for microbial quality. the tests include microbial enumeration tests to. Microbial Limit Test Ppt.
From www.slideshare.net
Microbial limit tests I.P By Dr.P.Srinivas Jangaon institute of pharm… Microbial Limit Test Ppt this document describes the microbial limit test, which includes tests to quantify and qualify microorganisms in. when we have microbiological limits that include the sampling plan, analytical procedures, and the define action or. this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. a presentation on microbial limit test for pharmaceutical. Microbial Limit Test Ppt.
From pacificbiolabs.com
Microbial Limits Test Pacific BioLabs Microbial Limit Test Ppt this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. a presentation on microbial limit test for pharmaceutical products, including total viable count, pathogen detection and media types. microbial limit test is performed to determine whether drug products comply with an established specification for microbial quality. this document describes the microbial. Microbial Limit Test Ppt.
From www.slideserve.com
PPT What Is Microbial Limit Test? PowerPoint Presentation, free Microbial Limit Test Ppt this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. this document summarizes a dissertation report submitted by ashish diwakar on microbial limit testing. a presentation on microbial limit test for pharmaceutical products, including total viable count, pathogen detection and media types. this document describes the microbial limit test, which includes. Microbial Limit Test Ppt.
From www.studypool.com
SOLUTION Microbial limit test Studypool Microbial Limit Test Ppt microbial limit test is performed to determine whether drug products comply with an established specification for microbial quality. this document describes the microbial limit test, which includes tests to quantify and qualify microorganisms in. a presentation on microbial limit test for pharmaceutical products, including total viable count, pathogen detection and media types. this document summarizes a. Microbial Limit Test Ppt.
From www.slideserve.com
PPT Practical aspects of Microbiological Testing and handling of OOS Microbial Limit Test Ppt when we have microbiological limits that include the sampling plan, analytical procedures, and the define action or. this pdf document provides tests for estimating the number and types of microorganisms in pharmaceutical. this document describes the microbial limit test, which includes tests to quantify and qualify microorganisms in. this document summarizes a dissertation report submitted by. Microbial Limit Test Ppt.